1. What is the surface and surface area?
The surface is the solid and the surrounding environment, especially the part where the liquid and gas interact with each other; the size of the surface, ie the surface area. The surface area can be increased by particle division (reducing particle size) and pore formation, and can also be reduced by sintering, melting and growth. ?
?
2. What is the specific surface area? Why is the surface area so important?
The specific surface area of ​​English is specific? surface? area, which refers to the total area of ​​a unit mass of material. There are two types of external surface area and internal surface area. The international standard unit is m2/g. ?
Surface area is a means and means by which a solid interacts with the surrounding environment, particularly liquids and gases. Generally have the following three functions:
1) Effect between solid-solid: manifested as automatic bonding, fluidity (sand), compression plasticity, etc. ?
2) Effect between solid and liquid: manifested as infiltration, non-infiltration, adsorption capacity, and the like. ?
3) Effect between solid and gas: manifested as adsorption, catalytic ability and the like. ?
3. What is a hole?
According to the definition in ISO15901, different pores (micropores, mesopores and macropores) can be regarded as pores, channels or cavities in solids, or spaces between solid particles forming beds, compacts and agglomerates ( Such as cracks or voids).
4. What are open and closed holes? 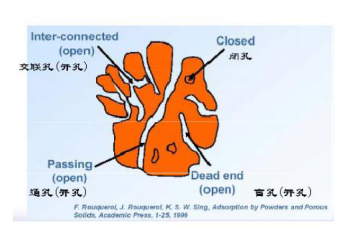
?
The cavities and channels that communicate with the outside in the porous solid are called open cells, including cross-linked holes, through-holes, and blind holes. The surface area of ​​these channels can be analyzed by gas adsorption. ?
In addition to the measurable pores, there may be some pores in the solid which are not in communication with the outer surface and which are not infiltrated by the fluid and therefore are not within the range of gas adsorption or mercury intrusion measurements. A hole that does not communicate with the outside is called a close?pore. ?
Open cells and closed cells are mostly formed during the preparation of porous solid materials, and sometimes can be formed during post-treatment. For example, high-temperature sintering can make open cells into closed cells. ?
?
5. What is porosity?
Porosity refers to the surface features whose depth is greater than the width and is generally characterized by the pore size and its distribution and total pore volume. ?
6. What is a porous material?
A porous material is a material that forms a network structure from pores that are interpenetrated or closed, and the boundaries or surfaces of the pores are composed of pillars or plates. The porous material may be in the form of a fine or coarse powder, a pressed body, an extruded body, a sheet or a block. Its characterization typically involves the determination of pore size distribution and total pore volume or porosity. In some cases, it is also necessary to examine the pore shape and flowability, and to measure the inner and outer surface areas. ?
?
7. What is the real surface like?
Cubes and spheres are the simplest ideal models for mathematical calculations. For a L?cm cube with a side length, the surface area is 6L2?cm2.
But in reality, the ideal geometry in mathematics does not exist at all, because all real surfaces are seen under the microscope, they are all flawed, and they are uneven. If you have a "super microscope", you can see how rough the surface is, not only because of voids, holes, steps and other non-ideal conditions, but also because of the distribution of atoms or molecular orbitals. These surface irregularities always create a larger real surface area than the corresponding theoretical area. ?
?
8. What are the factors that affect the surface area?
Factors affecting the surface area include particle size (particle size) and particle shape (grain shape) as well as pore content. Imagine a real cube that is one meter long and is cut into small cubes of one micron (10 -6 μm). This will produce 1018 particles.
The area exposed by each particle is 6x10-12 square meters (m2), and the total area contributed by all particles is 6x106?m2. A million-fold increase in this exposed area compared to uncut materials is typical of ultra-fine powders having a large surface area. In addition to the particle size, the particle shape also contributes to the surface area of ​​the powder. In all geometries, the sphere has the smallest area/volume ratio, but a string of atoms will have the largest area/volume ratio if only bonded along the chain axis. All particulate matter has a geometric shape and thus has a surface area between the two extremes. The effect of particle shape on surface area is readily seen by comparing two surface areas of particles having the same composition and the same mass, but spherical and cubic, respectively. It is calculated that the cube area is larger than the sphere area with the same particle weight. Because of the difference in particle size, grain shape, and porosity, the range of specific surface area can vary greatly, but the effect of pores tends to completely obliterate the effects of particle size and external shape factors. A powder consisting of 0.1 micron radius spherical particles with a density of about 3 g/cm3? has a specific surface area of ​​about 10 m2/g, while a similar particle with a 1.0 micron radius is 10 times smaller than the surface; but if the same 1.0 micron radius particles contain a large amount The pores may have a specific surface area exceeding 1000 m2/g. This clearly indicates the important contribution of the pore to the surface area. ?
?
9. Is the calculated surface area value on the particle size analyzer accurate?
Although the particle shape can be assumed to be a regular geometry, in most cases it is irregular, but the current popular particle size measurement method is based on the "equivalent sphere volume". If you try to measure the specific surface by means of particle size measurement methods (including laser diffraction, light scattering, electric field sensitivity, sedimentation, transmission, sieving and electron microscopy), due to grain shape, surface irregularities and pores The effect of the degree will be slightly smaller than the true value, or even more than 1000 times. Therefore, calculating the surface area from the particle size can only establish a low limit by the absolute assumption of a spherical or other regular geometry. ?
?
10. What are the types of holes?
As a porous material, an industrial catalyst or carrier is a collection of particles having a developed pore system. Generally, a certain atom (molecular) or ion forms a nanoporous crystal containing micropores according to the crystal structure rule; and due to the difference in preparation chemical conditions and chemical composition, several crystal grains can be aggregated into micron-sized particles of different sizes. And then industrially shaped into larger agglomerates or aggregates of particles with different geometric shapes. ?
Different preparation methods produce different pore structures. Such as, 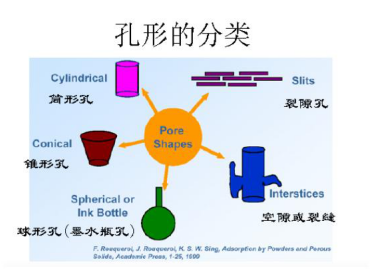 The pore structure of the high-temperature sintered or extruded porous solid is random; and the colloid precipitates, shrinks, and ages in the water-filled primary structure, producing characteristic microporous structures (typical examples such as cement and gypsum). ?
The pore structure of the high-temperature sintered or extruded porous solid is random; and the colloid precipitates, shrinks, and ages in the water-filled primary structure, producing characteristic microporous structures (typical examples such as cement and gypsum). ?
Zeolites and molecular sieves have a stable crystal structure, and the pores inside thereof are uniform and regular shapes composed of pores, slits or cages in the crystal. Inside the zeolite, the cage is connected by a window of 0.4?–?1 nm in diameter. A cage can be thought of as a spherical hole. ?
Therefore, the pore structure in the actual volume is complex and consists of different types of pores. At the molecular level, the inner surfaces of the pores are almost all matte. However, we can start with a few basic types (pictured) and then build various combinations of them. ?
The most typical is a cylindrical hole (cylindrical hole), which is a basic model for the calculation of the hole distribution. ?
Spherical or polyhedral particles that are extrusion cured but not yet sintered are mostly tapered holes (wedge holes, pyramidal spaces). ?
A fracture hole is a space formed by contact or stacking between particles. This model is also the basis for the calculation of swelling and agglomeration. ?
The ink bottle holes have a neck. The aperture is the neck of the larger aperture, so the ink bottle aperture can also be seen as a combination of a spherical aperture and a cylindrical aperture. The pores of the zeolite type are stable, but are controlled by the "neck", which can be regarded as an intermediate state between the cylindrical hole and the ink bottle hole. ?
?
11. How is the hole width classified?
According to the definition and classification of the International Association of Pure and Applied Chemistry (IUPAC) in 1985, the pore width is the pore diameter (to the cylindrical pore) or the distance between two opposing pore walls (for the fracture pore). therefore,?
(i)? Micropore refers to a hole with an internal pore width of less than 2 nm; 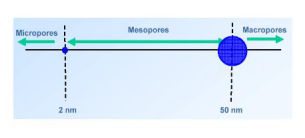
(ii) Mesopores are holes with a width between 2 nm and 50 nm;
(iii)? Macropore is a hole with a pore width greater than 50 nm. ?
In 2015, IUPAC? subdivided and supplemented the aperture classification, ie?
(iv) nanopore: including micropores, mesopores and macropores, but with an upper limit of only 100 nm;
(v) ultramicropore: narrow micropores with a pore width of less than 0.7 nm;
(vi) Supermicropore: A wider micropore with a pore width greater than 0.7 nm.
?
12. What are the types of surface and pore size analysis methods?
These methods include gas adsorption, mercury intrusion, electron microscopy (SEM or TEM), and small-angle X-ray scattering (SAXS).
And small angle neutron scattering (SANS) and the like. In 2010, the United States Dispersed Technology Corporation (DT) and the United States Conta Instruments also jointly developed the electroacoustic electro-vibration method, and the Occhio Company of Belgium developed the image method for large hole analysis. In general, each method has its application limitations in pore size analysis. ?
Gas adsorption is the most common method for different methods of pore size characterization, as its pore size measurement ranges from 0.35 nm to over 100 nm, covering all micropores and mesopores, and even extending to macropores. In addition, the gas adsorption technology is easy to operate and low in cost compared to other methods. If the gas adsorption method is combined with the mercury intrusion method, the pore size analysis range can cover from about 0.35 nm to 1 mm. Gas adsorption is also the best way to measure all surfaces, including irregular surfaces and the area inside the openings. ?
13. What is adsorption? What is the difference between it and absorption?
The gas and liquid on the solid surface are automatically aggregated on the solid surface in order to reduce the surface energy. The phenomenon that the concentration of a gas or liquid on a solid surface is higher than its bulk concentration is called surface adsorption of a solid. The process of adsorbing surrounding gas molecules across the solid surface is called gas adsorption. Monitoring the gas adsorption process has proven to provide a wealth of useful information about solids characteristics. ?
When the molecules of the adsorbed material penetrate the surface layer and enter the structure of the loose solid, this process is called absorption. Sometimes it is difficult or even impossible to distinguish the difference between adsorption and absorption, so that the more convenient or more widely used term sorption encompasses both adsorption and absorption, and the resulting Term: sorbent, sorbate and sorbate or sorptive. ?
When adsorption is used to represent a process, its corresponding inverse process is desorption. During the desorption process, due to the thermal motion of the molecules, the energetic molecules can break away from the binding force and leave the surface, and the amount of adsorption gradually decreases.
The terms "adsorption" and "desorption" were later used as adjectives to indicate the experimental study of the amount of adsorption, ie the adsorption curve (or point) or the desorption curve (or point). When the adsorption curve and the desorption curve do not coincide, adsorption hysteresis occurs. ?
?
14. What is the nature of adsorption?
?
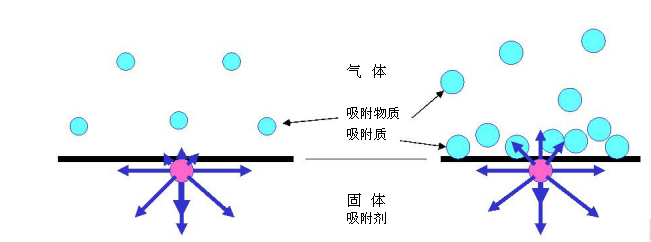
Everything is made up of molecules, which form the basis of the molecule. Gaseous atoms and molecules move freely. In contrast, in the solid state, atoms are in a fixed position due to electrostatic attraction between adjacent atoms. However, the outermost layer (or surface) of the solid has fewer adjacent atoms around the inner layer of atoms. The imbalance of the force of this outermost atom leads to the generation of surface energy. The atoms on a solid surface, like liquids, are not uniformly stressed, but unlike liquid surface molecules, they can move, but are positioned. Therefore, most solids have a higher surface energy than liquids. To compensate for this electrostatic gravitational imbalance, surface atoms adsorb gas molecules in the surrounding air. ?
???
15. What are adsorbents, adsorbates, adsorbed materials and adsorption spaces?
In general, adsorption is defined as the phenomenon of enrichment of molecules, atoms or ions in the vicinity of an interface. In the case of a gas/solid system, adsorption occurs on structures adjacent to the solid surface. The adsorbed solid material is called adsorbent; the substance in the adsorbed state is called adsorbate; the adsorbent in the mobile phase, but the same composition as the adsorbate is called adsorbor . The adsorption space refers to the space occupied by the adsorbate. The adsorption process is physical adsorption or chemical adsorption. ?
The adsorption system consists of three zones: solid, gas and adsorption space (eg, adsorption layer). The amount of content in the adsorption space is the amount adsorbed. The amount of adsorption depends on the volume, mass and adsorption space. ?
16. What is physical adsorption and chemisorption?
?
The adsorption mechanism of gas molecules on solid surfaces is extremely complex, including physical adsorption and chemical adsorption. ? 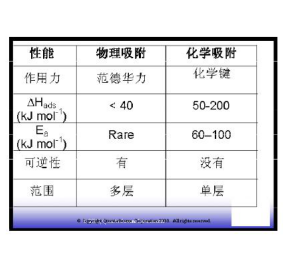
The adsorption produced by the intermolecular force (van der Waals force) is called physical adsorption. Physical adsorption is a common phenomenon that exists on the surface of a solid (adsorbent) that is brought into contact with the adsorbed gas (adsorbed material). The intermolecular forces involved are of the same type, for example, causing defects in the actual gas and condensation of the vapor. In addition to attracting dispersive forces and repulsive forces at close range, specific intermolecular interactions (eg, polarization, field-dipole, field) typically occur due to the specific geometry of the adsorbent and adsorbent species and the electronic properties of the outer layer. The quadrupole moment of the gradient). ?
Any molecule has a force, so physical adsorption is not selective, activation energy is small, adsorption is easy, and desorption is easy. It can be monolayer adsorption and multi-layer adsorption. ?
The adsorption caused by the formation of chemical bonds between molecules is called chemical adsorption; it is selective, the activation energy is large, the adsorption is difficult, and the desorption is difficult, and a higher temperature is often required. Chemical adsorption must be monolayer adsorption. ?
Actual adsorption may have both physical adsorption and chemical adsorption; first physical adsorption and then chemical adsorption. The amount of adsorption can be measured by the volume of the adsorbed substance (adsorbed matter) per unit mass of sample (adsorbent) at standard atmospheric pressure, and can be expressed in ml/g? or ?cc/g?@STP?. ?
At low temperatures, physical adsorption occurs mainly, and possible chemical adsorption occurs at high temperatures (specific reactions occur). The whole process involves high vacuum, low temperature, high temperature, high precision vacuum measurement, and the valve is set according to a preset procedure. Problems such as automatic switching. ?
? ? ?
17. What is the physical adsorption process of mesoporous materials?
According to the IUPAC report released in 2015, there are three different stages in physical adsorption that occur on mesoporous materials:
1) Monolayer?multilayer: All adsorbed molecules are in contact with the surface layer of the adsorbent.
2) Multilayer? Adsorption: The adsorption space contains more than one layer of molecules, so that not all of the adsorbed molecules are in direct contact with the surface of the adsorbent. In the mesopores, multiple layers of adsorption are followed by agglomeration that occurs in the pores. ?
Capillary? (or?pore)? Condensation: A phase in which a gas condenses into a liquid phase in a pore when the pressure p is less than its saturation pressure p0. Capillary condensation reflects in a limited
3) Gas-liquid phase changes occurring in the volume system. The term "capillary (or pore) agglomeration" cannot be used to describe the micropore filling process because no phase change between gas and liquid is involved in the micropores. ?
18. What is a gas adsorption isotherm?
If the absolute temperature, pressure and the action of the gas (adsorbate) and the surface (adsorbent) are constant, the amount of adsorption on a particular surface is constant. Since the amount of gas adsorbed by a solid surface is a function of temperature, pressure, and affinity or energy, we can plot the amount of adsorption per unit weight of adsorbent at equilibrium temperature at a constant temperature. The curve of the amount of adsorption versus pressure at a constant temperature is the adsorption isotherm of a particular gas-solid interface. ?
19. How to analyze the specific surface using the principle of gas adsorption?
The surface area per unit weight of the solid porous material (i.e., specific surface area) is an important physical parameter. The real surface includes irregular surfaces and internal surfaces of the holes. Their area cannot be calculated from particle size information, but can be determined by adsorbing an inactive or inert gas at the atomic level. The amount of gas adsorbed is not only a function of the total amount of exposed surface, but also (i)? temperature, (ii) gas pressure, and (iii) a function of the intensity of the reaction between the gas and the solid. Because most of the interaction between gas and solid is weak, in order to cause it to adsorb fairly enough to cover the entire surface, the surface must be sufficiently cooled to the boiling temperature of the gas. As the gas pressure increases, the amount of surface adsorption increases in a non-linear manner. However, when the gas completely covers the surface with one atomic thickness (monolayer gas), the adsorption of cold gas does not stop! As the relative pressure increases, excess gas is adsorbed to form a "multi-molecular layer" which may further liquefy to fill the entire pore.
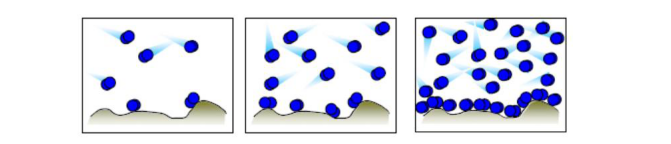
In order to achieve the above objectives, the sample is first vacuum degassed to clean the surface of the sample; if nitrogen is used as the molecular probe (ruler), the sample is then weighed together with the sample tube and placed in liquid nitrogen (-273 ° C). The controlled amount of nitrogen that has been metered by the pressure sensor is controlled to record the amount of adsorption of the sample. The process is quite complicated and lengthy. After obtaining the data of the saturated adsorption amount of the sample under different pressures, the desired result is calculated by an empirical formula (model) determined by the nature of the sample. ?
To make an incomplete analogy: to measure the area of ​​a room, but in addition to many basketballs do not have the right ruler, and the diameter and cross-sectional area of ​​the basketball is known. So, before measuring the area of ​​the house, first move the furniture placed in the house, then throw the basketball into the room. The number of thrown in can be controlled and calculated. When the basketball is covered with the house, we will basketball. Multiply the cross-sectional area by the number of basketballs thrown in to estimate the area of ​​the room. In the same way, then throwing basketball until the room is filled with basketball until the roof, we can infer the size of the room. The physical adsorber is designed to achieve this entire process.
KNM5 Series Moulded Case Circuit Breaker
KNM5 series Moulded Case Circuit Breaker is MCCB , How to select good Molded Case Circuit Breaker suppliers? Korlen electric is your first choice. All moulded Case Circuit Breakers pass the CE.CB.SEMKO.SIRIM etc. Certificates.
Moulded Case Circuit Breaker /MCCB can be used to distribute electric power and protect power equipment against overload and short-current, and can change the circuit and start motor infrequently. The application of Moulded Case Circuit Breaker /MCCB is industrial.
Korlen electric also provide Miniature Circuit Breaker /MCB. Residual Current Circuit Breaker /RCCB. RCBO. Led light and so on .
KNM5 series Molded Case Circuit Breaker,Small Size Molded Case Circuit Breaker,Electrical Molded Case Circuit Breaker,Automatic Molded Case Circuit Breaker
Wenzhou Korlen Electric Appliances Co., Ltd. , https://www.zjmotorstarter.com